(reprinted from here)
How to Prevent a Global Aging Crisis
July 17, 2010 by David Despain
A handful of forward-thinking biogerontologists has joined together to offer a new direction for aging intervention. Their commentary, published July 14 in Science Translational Medicine, presents the case for preventing what the scientists call an “unprecedented global aging crisis”—a sharp rise in the numbers of retired elderly in developing and industrialized nations across the world.
Red. In other words all modern societies (specifically US, Europe, Japan) will experience an unbearable societal stress, in a world that is essentially living beyond it’s sustainability footprint to begin with, because of a demographic accident. The consequences of having “too many old people” will be discrimination, exclusion and marginalization (and yes if you are reading this article that almost certainly includes you) if not much much worse. And all this will occur in a world where there will be radically less jobs 1, 2, 3, so old people will be the last to get any.)
From both a humane and economic standpoint, a world with too many sick elderly has grim consequences and outrageous costs. It’s time to fund research for prevention, slowing, or even reversal of the biological damage caused by simply living, which manifests as age-related diseases such as Alzheimer’s, cardiovascular disease, and cancer.
Currently, senescence research receives only a slim morsel of the researching-funding pie. Of the entire National Institutes of Health $28 billion budget, only about 0.1 percent (about $10 million) has been allocated for biological aging research, the scientists write. Of that $10 million morsel, only crumbs will make it to actual clinical studies on late-life aging interventions—despite the large economic and social implications of an increasing ratio of older to younger people.
The amounts of money which is ‘economically dormant’ sitting in pension funds numbers well in the tens of trillions. If society (pension funds) can either find compelling and fair reasons not to pay this money to the tsunami of pensioners (because they aren’t old anymore) then we can solve a demographic problem using an affordable engineering solution. How do we do this? We do by – as a society – rewarding insurers with tax incentives if they increase the over-all health of their clients with sensible measures. Anytime a medical insurer condones behavior or states in their clients that involves voluntary bad health, this results in some sort of punitive tate tax consequence for the insurer; likewise if the medical insurer creates conditions for the client that increase ‘over all’ health of clients, this results in a tax break for the insurer. So if medical insurance companies receive the ultimate tax break, they maximize over all health of their clients by doing whatever it takes to make clients young (or resilient) again. Now replace the word ‘medical insurers’ by ‘pension funds’ and you incentivize health itself – and thereby research for tangible treatments versus aging i.e. radical life extension.
Aging manifests itself as “a progressive, roughly synchronous rise in the incidence of disease, disability, and death from chronic diseases, beginning after midlife [examples in “Chronic diseases and aging” chart] and suggests a causal—rather than a casual—relationship,” the authors write.
[+]
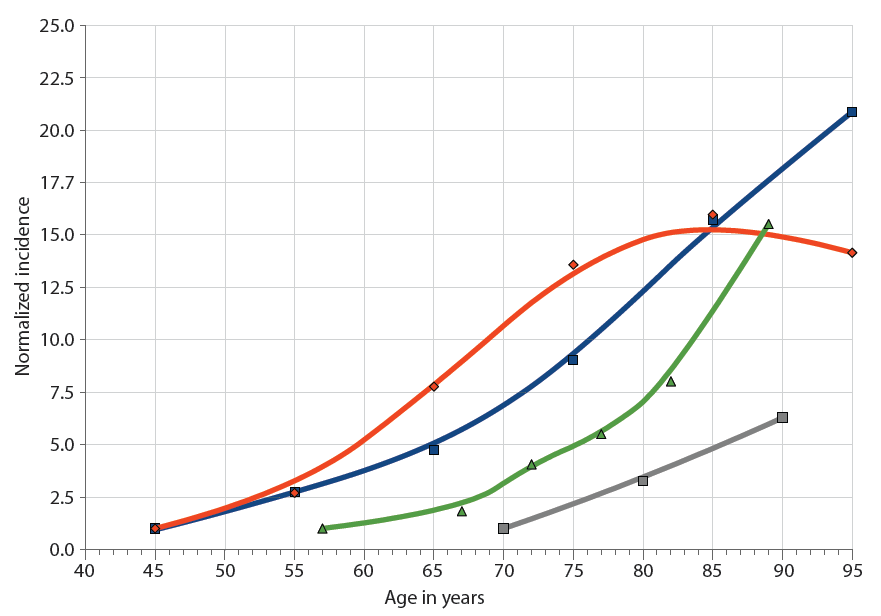
Chronic diseases and aging. The incidence of major chronic diseases increases with age: cardiovascular disease (blue squares), cancer (red diamonds), AD (gray squares), and influenza-associated hospitalization (green triangles). Incidence rates are normalized to the first data point. (Chart: AAAS)
Research Roadmap for Optimizing and Postponing Aging
A co-author of the paper, Aubrey de Grey, a biomedical gerontologist and author of the popular science book Ending Aging, sums up the case for a new way forward: “We argue for a more balanced approach to the quest for interventions to postpone age-related ill health.” De Grey, the Chief Science Officer of the SENS (Strategies for Engineering Negligible Senescence) Foundation (SENSF), Sunnyvale, California, is no stranger to the problems of finding funding for research, which is why he and his co-authors decided to write the commentary.
“There are no surprises there,” he says. “It’s all about the fact that biology is irreducibly expensive. In particular, as more and more of our research programs move from the cell culture stage into live mice, the expense rises sharply.”
The high cost of anti-aging research, de Grey says, is the greatest roadblock in the scientists’ roadmap, which consists of three intervention strategies pursued by researchers at SENSF and other organizations, including the International Longevity Center in New York, and the Andrus Gerontology Center, University of Southern California of Los Angeles):
* Reduce exposure to environmental toxins and ameliorate other risk factors through improved public health.
* Slow down damage to metabolic pathways, which contributes to age-related changes.
* Develop a more broadly conceived regenerative medicine to embrace the repair, removal, or replacement of existing aging damage or its decoupling from its pathological sequelae.
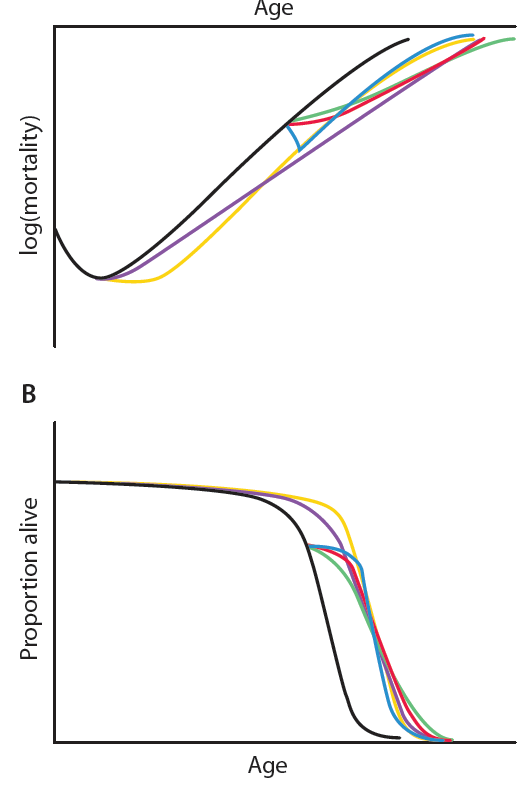
Postponing degeneration. This illustration compares the trajectories of the proposed modalities of intervention necessary to achieve a 7-year average postponement of the onset of age-related degeneration, depicted in terms of (A) an exponential rise in mortality rates and (B) survival. The black trajectories indicate scenarios if no interventions are applied. Nutritional and other public health interventions would need to be applied aggressively and from an early age (ideally prenatally) (yellow trajectories). Metabolic interventions applied from an early age would suffice even if they only mildly slowed the rate of accumulation of aging damage (purple). Metabolic interventions applied only from middle age would need to at least halve this rate—a daunting challenge (green). Late-onset regenerative therapies would postpone biological aging by substantially reversing the initial level of aging damage and then allowing it to continue as normal, but would also be challenging to implement comprehensively enough (blue). A combination of more modest implementations of the late-onset metabolic and regenerative approaches seems most tractable and could lead to an equal or greater extension of healthy productive life (red). (Chart: AAAS)
The authors highlight a 2006 article in The Scientist, co-authored by the former biomedical gerontologist Robert N. Butler of the International Longevity Center in New York: “The relative potential of these interventions and their combination is portrayed in [the “Postponing degeneration” chart], presented in terms of their ability to deliver a 7-year postponement of age-related degeneration.”
Unfortunately, most biogerontologists see aging-intervention strategies as a considerable deviance from mainstream thinking, which is based on the notion that aging is a certainty and that pursuit of any kind of “fountain of youth” or life-extension therapies will only end in failure.
But de Grey is not swayed by the skeptics. He says perspectives are changing: “Five of the other authors [of the paper] are among the absolute top tier of biogerontologists, whose views are universally respected in the field. Their voice here will make a huge impact on thinking about the issue, both within the field and beyond. ”
“The surprising conclusion from the past two decades of research on biological aging is that aging is plastic,” the authors of the paper state. “Within a species, maximum life span is not fixed, but can be increased by dietary manipulation (particularly calorie restriction) or genetic manipulation.”
But a new world of indefinite lifespans has also raised questions about potential population impacts. “Contrary to what is widely assumed, however, the net effect should be relatively minor,” the authors respond, reasoning that new human births have a greater effect on population than adding a fraction of life span to existing humans. “A policy of aging as usual will lead to enormous humanitarian, social, and financial costs. Efforts to avert that scenario are unequivocally merited, even if those efforts are costly and their success and full consequences uncertain. To realize any chance of success, the drive to tackle biological aging head-on must begin now.”
These new perspectives about the importance of biological aging research and how it should be carried out are welcome news for scientists researching pre-disease interventions and human regenerative-engineering therapies, and for those of us who would one day like the chance to share moments with our great-great-great-great grandchildren.
Normally the political caste has a charter to rule regarding their own postage stamp policies. Politicians do not deal with intangibles or remote contingencies. Politicians rule (pathologically) short term. Politicians get headaches with longterm. However, all politicians will they themselves have to live in this long term, and probably of above average pensions. Now what if those politicians had to look forward to a rather sinister, persecutary future, where they would be blamed for all of society’s ill’s – OR they would be living in that future indefinitely, beneficiaries of rather plush life extension treatments they themselves initiated? That would be a fascinating legacy for these politicians indeed, one for which they would not merely be lauded, but a future where they would be lauded for potentially quite a long time. All it takes is vision, and we all know that youth comes with significantly clearer eyes, yes?
The demographic and biomedical case for late-life interventions in aging. Sci. Transl. Med. 2, 40cm21 (2010). Supplementary Material (free full-text access)
In pursuit of the longevity dividend. The Scientist, 20: 28-36.
SENSF Solutions to Seven Causes of Aging Damage
Over the past decade, Aubrey de Grey has identified seven “major categories of molecular and cellular ‘damage’ that I believe we need to repair (or in some cases obviate) to rejuvenate the aged body comprehensively, and ways to implement that repair”:
1. Cell loss, tissue atrophy
2. Nuclear mutations
3. Mutant mitochondria
4. Death-resistant cells
5. Tissue stiffening
6. Extracellular aggregates
7. Intracellular aggregates
These interventions include repairing the body with stem cell therapies to combat cell loss, “suicide” gene therapy to act against death-resistant cells, and enzymes or vaccinations for cleaning out intracellular and extracellular “molecular garbage. ”
SENSF also directs research on small-molecule drugs that can act against spontaneous cross-links in the extracellular matrix, nuclear copies of the mitochondrial DNA to avert mitochondrial mutations, and complex combination therapy to suppress telomere elongation (telomeres are a repetitive DNA sequence protecting the end of chromosomes from degeneration, like the plastic tips on shoelaces), using a variety of stem-cell therapies to preempt cancer.
De Grey says the foundation’s priorities are long-term research commitments. In the coming few years, ”I believe the main benefits we can expect to see will come from public health advances, and possibly from drugs to optimize metabolism. These will act as a ‘bridge’ to allow more people to survive in a healthy state.”
Updates
* http://www.sens.org/node/1245
* PDF
[youtube=http://www.youtube.com/watch?v=u8CyNQDsass&hl=en_US&fs=1]
Thank you very much my friend, you are very kind in sharing this useful information with? others….
The details were such a blessing, thanks.